SIGNATERA™
A powerful ctDNA molecular monitoring tool offered exclusively in Switzerland by Unilabs
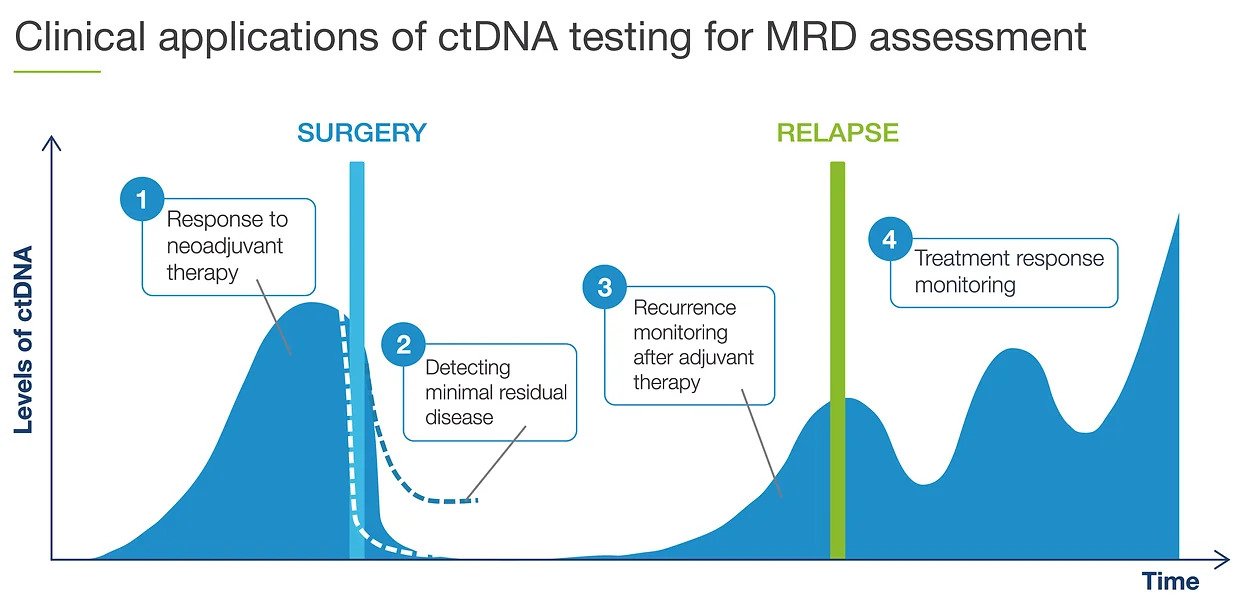
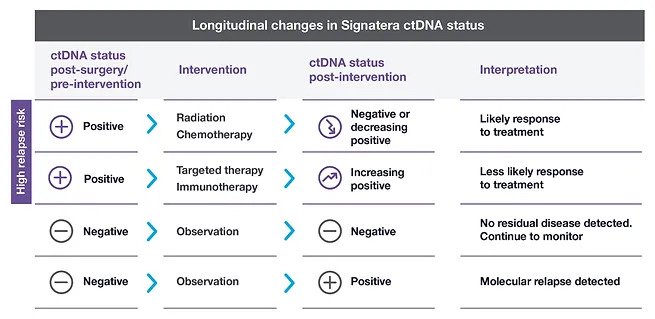
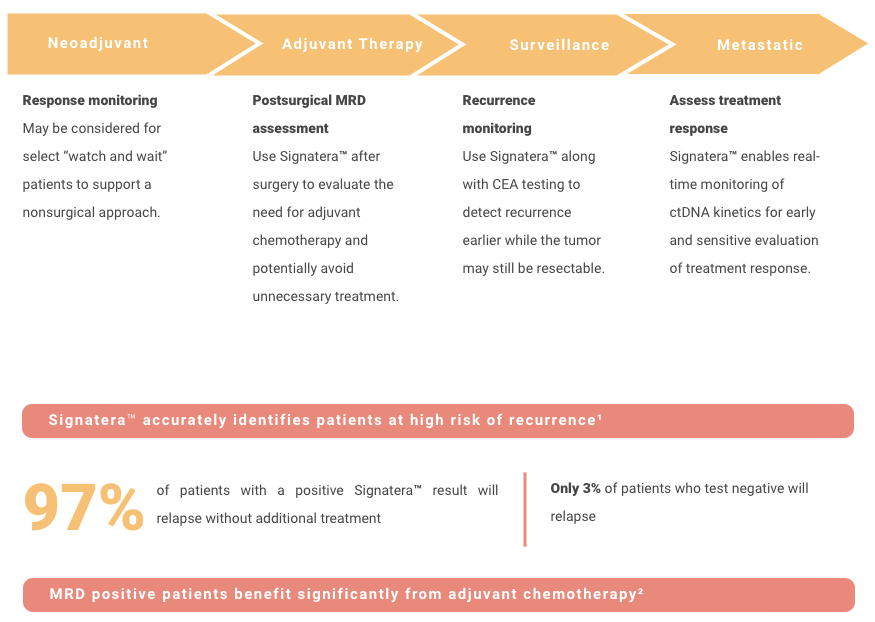
Adjuvant setting: Determines if the patient may have residual or recurrent disease
Use of Signatera after surgery to evaluate the need for adjuvant chemotherapy
Overview on the therapeutic scheme of the patient
Metastatic setting: Determines progression or response during treatment
Assess for MRD more accurately than current risk-assessment methods
Use Signatera to detect recurrence earlier while it may still be resectable, or to reduce false positive CEA results
 X
X 
Signatera™ is the first ctDNA molecular monitoring tool that detects molecular residual disease (MRD)
Cell free circulating tumour DNA (ctDNA) has emerged as a promising non-invasive cancer biomarker for monitoring disease status of cancer patients. It consists of short nucleic acid fragments released in the systemic circulation as a result of tumour cell apoptosis and/or necrosis, providing important infromation on the unique genomic profile of every neoplasia.
The ctDNA is the physiological tool that enables the early detection of tumour cells following cancer treatment or surgical intervention, namely molecular residual disease. Its assement is important when planning a course of treatment, evaluating its effectiveness, determining whether the cancer has returned, or making a prognosis.
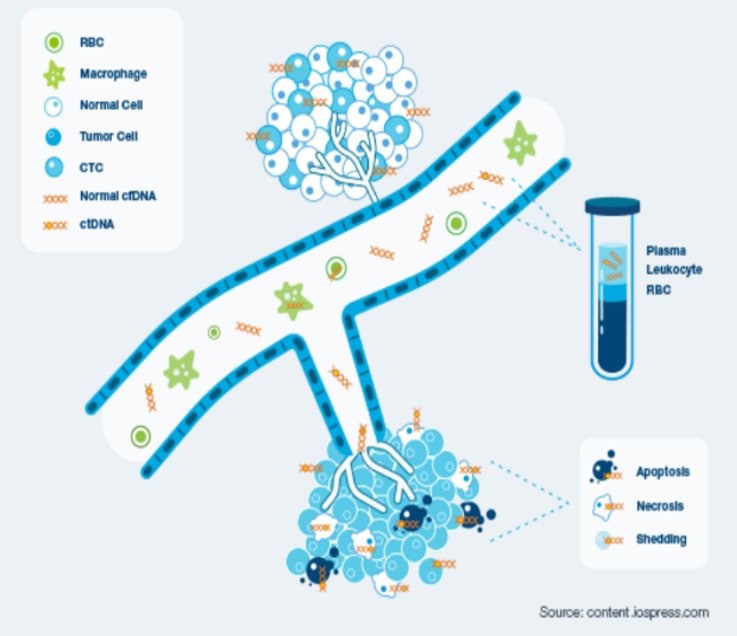
WHY IS CIRCULATING TUMOR DNA (ctDNA) INTERESTING FOR MRD ASSESSMENT?
-
Every patient’s tumor has a unique molecular profile and a dynamic evolution.
-
Cancer cells release circulating tumor DNA (ctDNA) into the bloodstream that reflects each tumor’s unique genetic fingerprint.
-
ctDNA is a powerful tool that can be used to measure and assess the absence or presence of MRD based on the tumor’s unique molecular profile.
-
Dynamic real-time biomarker: the normal half-life is less than an hour.
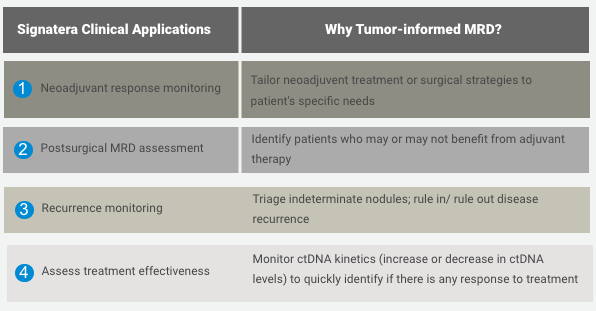
SIGNATERA™ IS TRULY PERSONALIZED
CUSTOM-BUILT ASSAY
-
Based on the unique mutation signature of each patient’s tumor
-
Identifies and tracks tumor mutations at the source
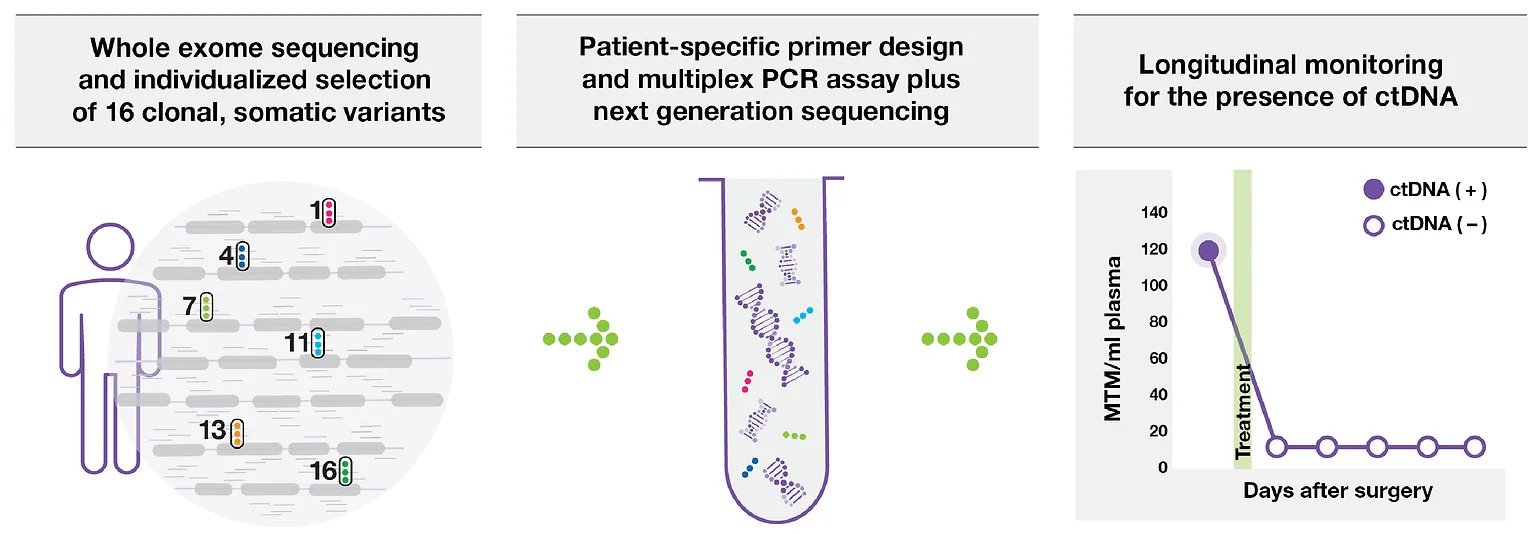
Once a personalized assay is designed, a patient’s blood can be used to accurately monitor for the presence or absence of the disease over time
TUMOR INFORMED
-
Tracks 16 clonal variants based on whole exome sequencing (WES) of tumor tissue and matched normal blood for each patient in order to identify specific DNA biomarkers of the tumour.
SENSITIVITY & SPECIFICITY
Signatera™ is optimized to detect extremely low quantities of ctDNA, and determines the presence or absence of molecular residual disease.
Ultra sensitive MRD Detection at VAFs as low as 0.01% by tracking 16 ctDNA specific markers (>99% of cases)
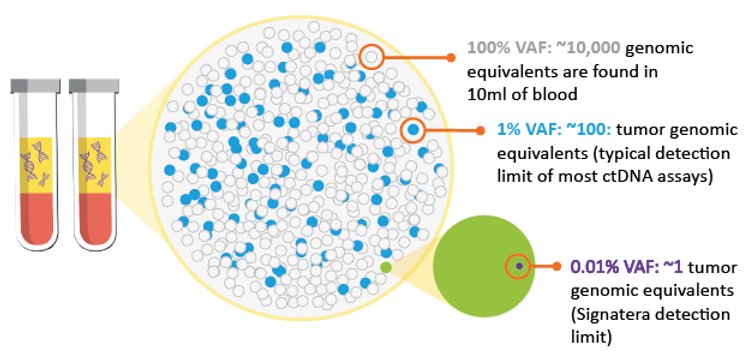
SUMMARY
-
Detects molecular residual disease at any point for greater confidence in clinical decisions.
-
The only custom ctDNA test that provides early knowledge with a >99.5% clinical test specificity.
-
Delivers higher sensitivity and specificity in detection of MRD versus conventional monitoring tools and static, liquid-biopsy panels.
-
Strongly predictive of eventual clinical relapse and poor clinical outcomes with a positive test result
-
Enables monitoring of molecular disease status at diagnosis and throughout the continuum of cancer care.
CLINICAL MANAGEMENT AND FOLLOW UP AFTER SIGNATERA™ TESTING
Colorectal Cancer (CRC)
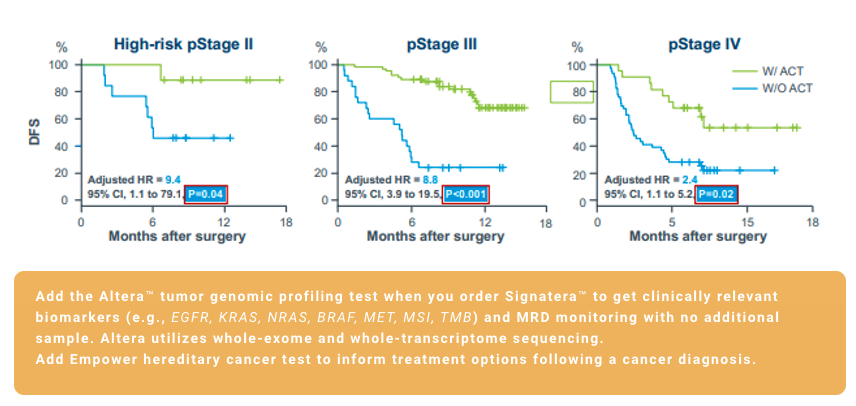
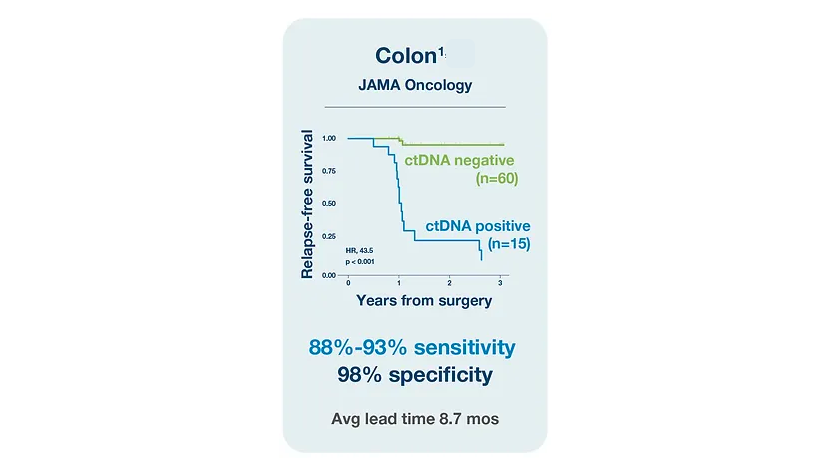
References
¹. Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124-1131. doi:10.1001/jamaoncol.2019.0528 2. Kotaka et al.
². Association of circulating tumor DNA dynamics with clinical outcomes in the adjuvant setting for patients with colorectal cancer from an observational GALAXY study in CIRCULATE-Japan. ASCO GI 2022
Bladder Cancer
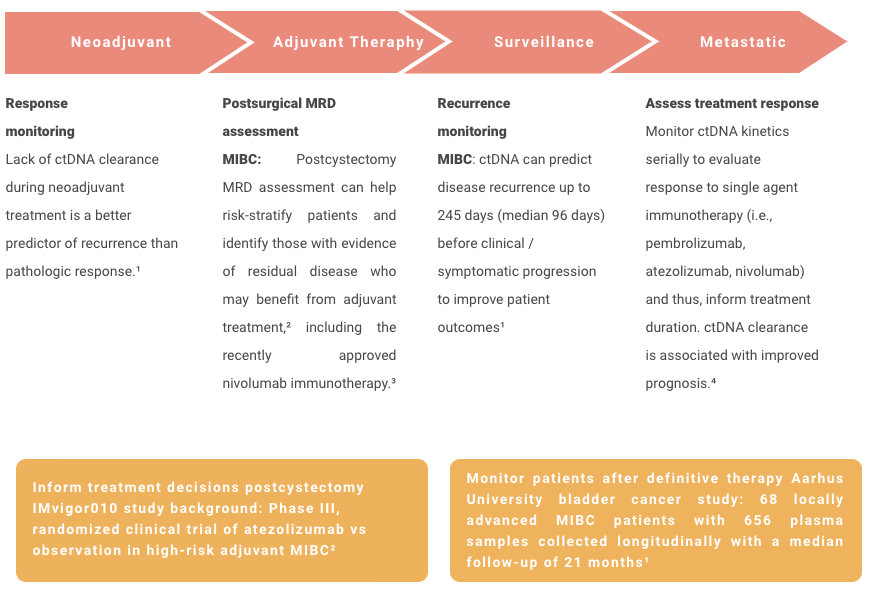
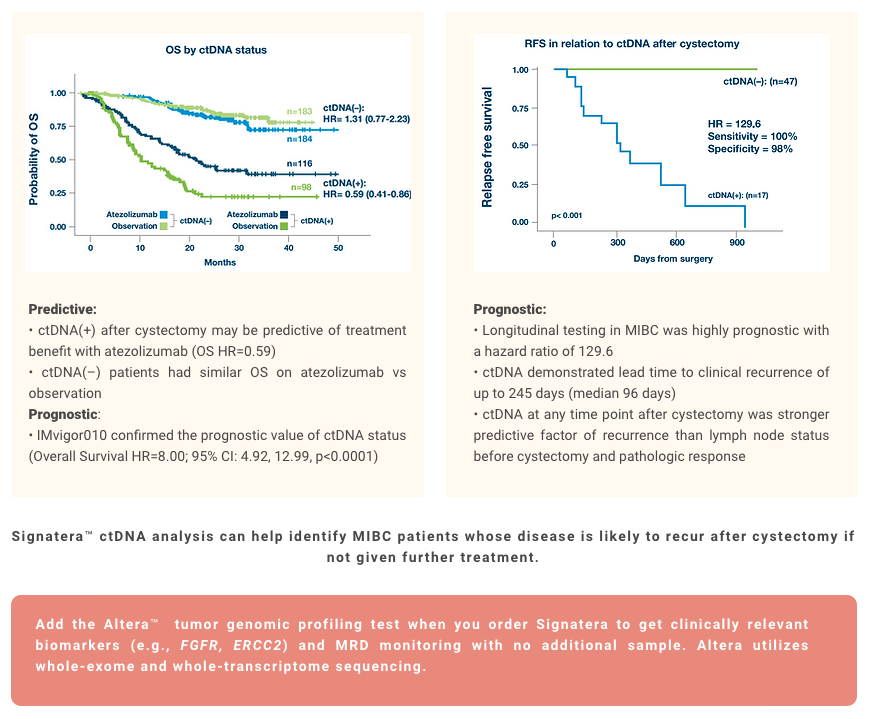
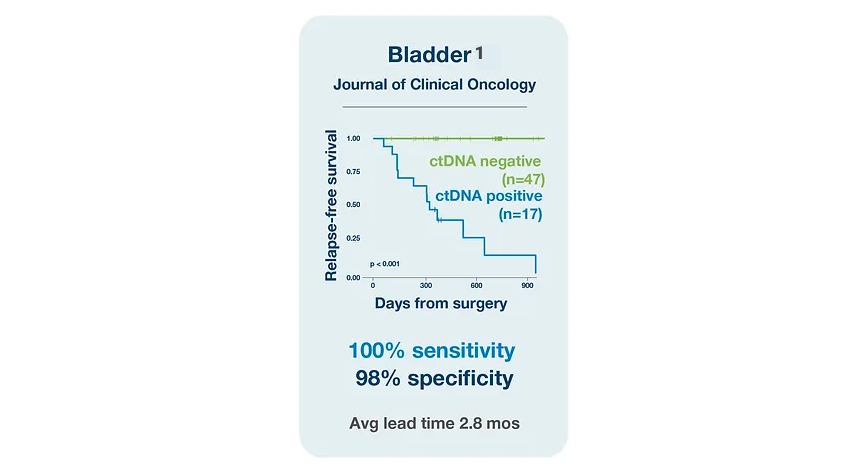
References
¹. Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol. 2019;37(18):1547-1557. doi:10.1200/JCO.18.02052
². Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432-437. doi:10.1038/s41586-021-03642-9
³. FDA approves nivolumab for adjuvant treatment of urothelial carcinoma. News release. Food and Drug Administration; August 20, 2021. Accessed November 2, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma
⁴. Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1:873-881.
doi:10.1038/s43018-020-0096-5
